| 1 | Hydrogen | H |
| 2 | Helium | He |
| 3 | Lithium | Li |
| 4 | Beryllium | Be |
| 5 | Boron | B |
| 6 | Carbon | C |
| 7 | Nitrogen | N |
| 8 | Oxygen | O |
| 9 | Fluorine | F |
| 10 | Neon | Ne |
| 11 | Sodium | Na |
| 12 | Magnesium | Mg |
| 13 | Aluminum | Al |
| 14 | Silicon | Si |
| 15 | Phosphorus | P |
| 16 | Sulfur | S |
| 17 | Chlorine | Cl |
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |
| 21 | Scandium | Sc |
| 22 | Titanium | Ti |
| 23 | Vanadium | V |
| 24 | Chromium | Cr |
| 25 | Manganese | Mn |
| 26 | Iron | Fe |
| 27 | Cobalt | Co |
| 28 | Nickel | Ni |
| 29 | Copper | Cu |
| 30 | Zinc | Zn |
Atomic Number Chart
| 31 | Gallium | Ga |
| 32 | Germanium | Ge |
| 33 | Arsenic | As |
| 34 | Selenium | Se |
| 35 | Bromine | Br |
| 36 | Krypton | Kr |
| 37 | Rubidium | Rb |
| 38 | Strontium | Sr |
| 39 | Yttrium | Y |
| 40 | Zirconium | Zr |
| 41 | Niobium | Nb |
| 42 | Molybdenum | Mo |
| 43 | Technetium | Tc |
| 44 | Ruthenium | Ru |
| 45 | Rhodium | Rh |
| 46 | Palladium | Pd |
| 47 | Silver | Ag |
| 48 | Cadmium | Cd |
| 49 | Indium | In |
| 50 | Tin | Sn |
| 51 | Antimony | Sb |
| 52 | Tellurium | Te |
| 53 | Iodine | I |
| 54 | Xenon | Xe |
| 55 | Cesium | Cs |
| 56 | Barium | Ba |
| 57 | Lanthanum | La |
| 58 | Cerium | Ce |
| 59 | Praseodymium | Pr |
| 60 | Neodymium | Nd |
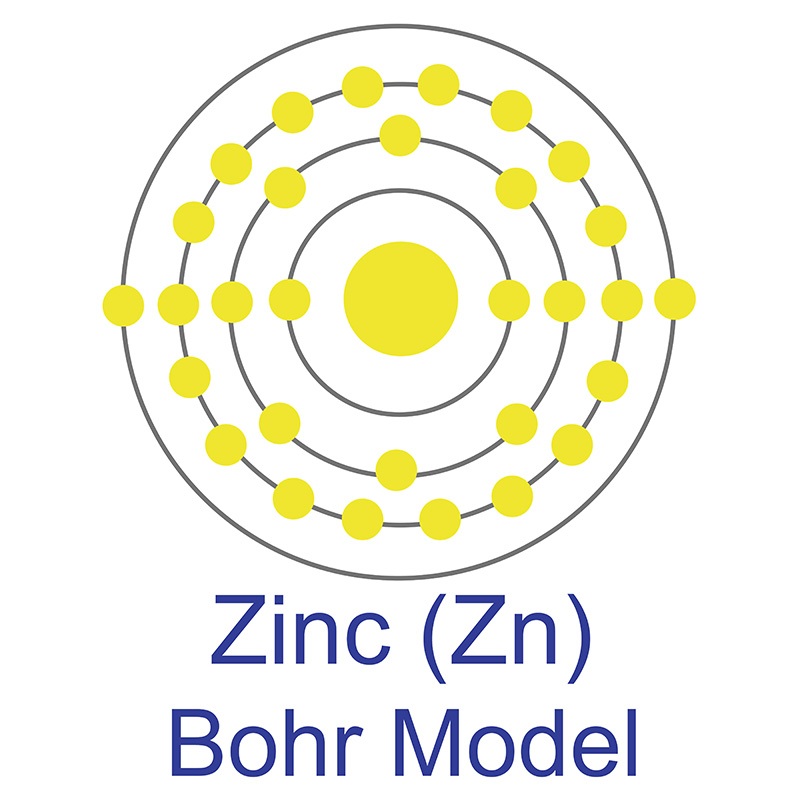
Atomic Number: 30: Symbol: Zn: Element Category: Transition Metal: Phase at STP: Solid: Atomic Mass amu 65.409: Density at STP g/cm3 7.14: Electron Configuration Ar 3d10 4s2: Possible Oxidation States +2: Electron Affinity kJ/mol — Electronegativity Pauling scale 1.65: 1st Ionization Energy eV 9.3941: Year of Discovery: unknown. Element Symbol Atomic Number Atomic mass Protons Electrons Carbon C 6 12.011 6 6 Oxygen O 8 15.999 8 8 Potassium K 19 19 Gold Au 79 196.96 79 79 Tin Sn 50 118.71 50 50 Zinc Zn 30 65.37 30 30 Zinc Zn 30 65.37 30 30 Cobalt Co 27 58.93 32 27 Boron B.
| 61 | Promethium | Pm |
| 62 | Samarium | Sm |
| 63 | Europium | Eu |
| 64 | Gadolinium | Gd |
| 65 | Terbium | Tb |
| 66 | Dysprosium | Dy |
| 67 | Holmium | Ho |
| 68 | Erbium | Er |
| 69 | Thulium | Tm |
| 70 | Ytterbium | Yb |
| 71 | Lutetium | Lu |
| 72 | Hafnium | Hf |
| 73 | Tantalum | Ta |
| 74 | Tungsten | W |
| 75 | Rhenium | Re |
| 76 | Osmium | Os |
| 77 | Iridium | Ir |
| 78 | Platinum | Pt |
| 79 | Gold | Au |
| 80 | Mercury | Hg |
| 81 | Thallium | Tl |
| 82 | Lead | Pb |
| 83 | Bismuth | Bi |
| 84 | Polonium | Po |
| 85 | Astatine | At |
| 86 | Radon | Rn |
| 87 | Francium | Fr |
| 88 | Radium | Ra |
| 89 | Actinium | Ac |
| 90 | Thorium | Th |
| 91 | Protactinium | Pa |
| 92 | Uranium | U |
| 93 | Neptunium | Np |
| 94 | Plutonium | Pu |
| 95 | Americium | Am |
| 96 | Curium | Cm |
| 97 | Berkelium | Bk |
| 98 | Californium | Cf |
| 99 | Einsteinium | Es |
| 100 | Fermium | Fm |
| 101 | Mendelevium | Md |
| 102 | Nobelium | No |
| 103 | Lawrencium | Lr |
| 104 | Rutherfordium | Rf |
| 105 | Dubnium | Db |
| 106 | Seaborgium | Sg |
| 107 | Bohrium | Bh |
| 108 | Hassium | Hs |
| 109 | Meitnerium | Mt |
| 110 | Darmstadtium | Ds |
| 111 | Roentgenium | Rg |
| 112 | Copernicium | Cn |
| 113 | Nihonium | Nh |
| 114 | Flerovium | Fl |
| 115 | Moscovium | Mc |
| 116 | Livermorium | Lv |
| 117 | Tennessine | Ts |
| 118 | Oganesson | Og |
Download a printable version of the Periodic Table of Elements in PDF format:
Atomic Number Of Zn-65
- Color: Basic / Advanced
- Black and White: Basic / Advanced
The following on-line games based on the Periodic Table of Elements are available:
- Element Flash Cards
- Element Hangman
- Element Matching
- Element Math

- Element Crossword Puzzles
- Element Concentration
- Element Balancing
- Element Word Scramble
The following paper-based activities are available:
- Element BINGO
Atomic Number Of Zn 65
- Element Word Search
In addition to the information contained within the Periodic Table of Elements, the following articles may be helpful if you are writing a report about an element or if you are making a model of an atom:
- How to calculate the number of protons, neutrons and electrons in an atom of an element
How To Find Atomic Number
- How to make a model of an atom
Atomic Number Of Zn
- How to draw an atom (video)
- How to read an electron configuration chart
Atomic Number Of Zinc Is 30
- A list of who discovered each element
The information on this site has been compiled from a number of sources.
For questions about this page, please contact Steve Gagnon.
